Identification of Drug Related Problems (DRPs) Use of Antibiotics in Pediatric Pneumonia Patients at General Hospital Bengkulu City
DOI:
https://doi.org/10.23917/pharmacon.v22i2.6672Keywords:
Antibiotics, DRPs, PCNE, Pediatrics, PneumoniaAbstract
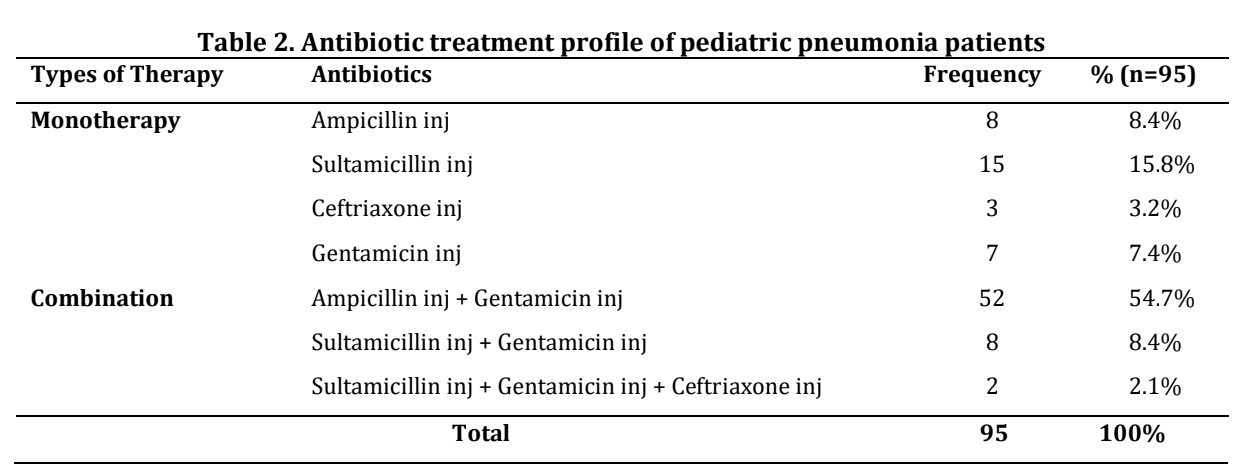
Pneumonia in pediatrics has a high mortality rate. Primary antibiotic therapy can increase antibiotic use and cause Drug Related Problems (DRPs). This study examines the treatment profile and identifies DRPs of antibiotic use in pediatric pneumonia patients at Ummi General Hospital, Bengkulu City. This study uses a cross-sectional approach method where data is collected retrospectively through patient medical records. The population includes all pediatric patients with the main disease community-acquired-pneumonia who were treated at the Ummi General Hospital, Bengkulu City during the July-December 2022 period. The sampling technique in this study was using purposive sampling. Data was analyzed univariately, and DRPs were identified using the Pharmaceutical Care Network Europe Foundation (PCNE) algorithm version 9.1 and analyzed descriptively. The treatment profile included ampicillin inj + gentamicin inj (54.7%), sultamicillin inj (15.8%), sultamicillin inj + gentamicin inj (8.4%), ampicillin inj (8.4%), gentamicin inj (7.4%), ceftriaxone inj (3.2%), and sultamicillin inj + gentamicin inj + ceftriaxone inj (2.1%). There were 367 cases in 95 patients. Cases of DRPs included overdose (27.8%), the duration of treatment is too short (22.9%), infrequent dose regimen (18.8%), adverse drug incidence (16.9%), underdose (10.1%), drugs not following the guidelines (1.9%), the duration of treatment is too long (1.1%), and therapeutic group duplication (0.5%). The study concluded that the most common treatment was ampicillin inj + gentamicin inj (54.7), with the most frequent DRPs being overdose (27.8%), the duration of treatment is too short (22.9%), infrequent dose regimen (18.8%), adverse drug incidence (16.9%), and underdose (10.1%).
Downloads
References
Abrogoua, D., Békégnran, C., Gro, B., Doffou, E., & Folquet, M. (2017). Assessment of a Clinical Pharmacy Activity in a Pediatric Inpatient Department in Cote D′ivoire. Journal of Basic and Clinical Pharmacy, 8(1), 15–19.
Al azmi, A., Ahmed, O., Al hamdan, H., Al garni, H., El zain, R. M., Al thubaiti, R. S., Aseeri, M., & Al Shaikh, A. (2019). Epidemiology of Preventable Drug-Related Problems (DRPs) Among Hospitalized Children at KAMC-Jeddah: a Single-Institution Observation Study. Drug, Healthcare and Patient Safety, 11, 95–103.
Astiti, P. M. A., Mukaddas, A., & Safarudin. (2017). Identifikasi Drug Related Problems (DRPs) pada Pasien Pediatri Pneumonia Komunitas di Instalasi Rawat Inap RSD Madani Provinsi Sulawesi Tengah. Jurnal Farmasi Galenika (Galenika Journal of Pharmacy), 3(1), 57–63.
Baxter, K. (2010). Stockley’s Drug Interactions. In Focus on Alternative and Complementary Therapies (9th ed.). Pharmaceutical Press. https://doi.org/10.1111/j.2042-7166.2001.tb02784.x
Birarra, M. K., Heye, T. B., & Shibeshi, W. (2017). Assessment of Drug-Related Problems in Pediatric Ward of Zewditu Memorial Referral Hospital, Addis Ababa, Ethiopia. International Journal of Clinical Pharmacy, 39(5), 1039–1046.
Bizuneh, G. K., Adamu, B. A., Bizuayehu, G. T., & Adane, S. D. (2020). A Prospective Observational Study of Drug Therapy Problems in Pediatric Ward of a Referral Hospital, Northeastern Ethiopia. International Journal of Pediatrics (United Kingdom), 2020, 1–6.
Black, K., Shalat, S. L., Freeman, N. C. G., Jimenez, M., Donnelly, K. C., & Calvin, J. A. (2005). Children’s Mouthing and Food-Handling Behavior in an Agricultural Community on the US/Mexico Border. Journal of Exposure Analysis and Environmental Epidemiology, 15, 244–251. https://doi.org/10.1038/sj.jea.7500398
California, S. H., Sinuraya, R. K., Halimah, E., & Subarnas, A. (2018). Perbandingan Efektivitas Ampisilin dengan Ampisilin-Gentamisin pada Pasien Balita dengan Pneumonia. Jurnal Farmasi Klinik Indonesia, 7(1), 52–58. https://doi.org/10.15416/ijcp.2018.7.1.52
Chua, E., & Hearsey, D. (2023). P24 Auditing antibiotic course lengths for the management of community-acquired pneumonia and hospital-acquired pneumonia against current NICE guidance. JAC-Antimicrobial Resistance, 5(2), 1–12.
Dinkes Provinsi Bengkulu. (2023). Profil Kesehatan Provinsi Bengkulu 2022. Dinas Kesehatan Provinsi Bengkulu.
DiPiro, J. T., Yee, G. C., Posey, L. M., Haines, S. . ., Nolin, T. . ., & Ellingrod, V. . . (2020). Pharmacotherapy : A Pathophysiologic Approach (11th ed.). McGraw-Hill Education.
FDA. (2023). Pediatric Drug Development: Regulatory Considerations-Complying With the Pediatric Research Equity Act and Qualifying for Pediatric Exclusivity Under the Best Pharmaceuticals for Children Act Guidance for Industry (Revision 1). Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/guidance-compliance-regulatory-information-biologics/biologics-guidances
Harris, M., Clark, J., Coote, N., Fletcher, P., Harnden, A., McKean, M., & Thomson, A. (2011). British Thoracic Society guidelines for the management of community acquired pneumonia in children: Update 2011. THORAX : Journal of the British Thoracic Society, 66(SUPPL. 2), 1–26. https://doi.org/10.1136/thoraxjnl-2011-200598
Hartini, L., & Ismiati. (2017). Kejadian Pneumonia Pada Batita Di Wilayah Kerja Puskesmas Sukamerindu Kota Bengkulu. Jurnal Media Kesehatan, 10(2), 102–204.
Katzung, B. G. (2018). Basic and Clinical Pharmacology (14th ed.). McGraw-Hill Education.
Katzung, B., Kruidering, M., & Trevor, A. (2019). Pharmacology Examination & Board Review (12th ed.). McGraw-Hill Education.
Kemenkes RI. (2022). Profil Kesehatan Indonesia. Kementerian Kesehatan Republik Indonesia.
Leopoldino, R. D., Santos, M. T., Costa, T. X., Martins, R. R., & Oliveira, A. G. (2019). Drug Related Problems in the Neonatal Intensive Care Unit: Incidence, Characterization and Clinical Relevance. BMC Pediatrics, 19(134), 1–7.
Majhi, A., Kundu, K., Adhikary, R., Banerjee, M., Mahanti, S., Basu, A., & Bishayi, B. (2014). Combination therapy with ampicillin and azithromycin in an experimental pneumococcal pneumonia is bactericidal and effective in down regulating inflammation in mice. Journal of Inflammation, 11(5), 1–17. https://doi.org/10.1186/1476-9255-11-5
Marcus, R., Paul, M., Elphick, H., & Leibovici, L. (2011). Clinical implications of β-lactam-aminoglycoside synergism: Systematic review of randomised trials. International Journal of Antimicrobial Agents, 37, 491–503. https://doi.org/10.1016/j.ijantimicag.2010.11.029
Martinez, M. N., Papich, M. G., & Drusano, G. L. (2012). Dosing regimen matters: The importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrobial Agents and Chemotherapy, 56(6), 2795–2805. https://doi.org/10.1128/AAC.05360-11
Mitul, M. T., Kastenschmidt, J. M., Sureshchandra, S., Wagoner, Z. W., Sorn, A. M., Mcllwain, D. R., Hernandez-Davies, J. E., Jain, A., de Assis, R., Trask, D., Davies, D. H., & Wagar, L. E. (2024). Tissue-specific Sex Differences in Pediatric and Adult Immune Cell Composition and Function. Frontiers in Immunology, 15(1373537), 1–16. https://doi.org/10.3389/fimmu.2024.1373537
Monica, C., S, A., & Dalilla, S. (2021). Evaluasi Drug Related Problems (DRPs) Antibiotik pada Pasien Pneumonia Rawat Inap Anak Rumah Sakit Umum Daerah Deli Serdang. Jurnal Farmasimed (Jfm), 3(2), 63–68.
Muenchhoff, M., & Goulder, P. J. R. (2014). Sex differences in Pediatric Infectious Diseases. Journal of Infectious Diseases, 209(SUPPL. 3), 120–126. https://doi.org/10.1093/infdis/jiu232
Nasution, E. S., Muchtar, R., & Syahputra, R. A. (2022). The Study of Drug-Related Problems in Pediatric Inpatients Utilizing Antibiotics in Universitas Sumatera Utara Hospital Medan, Indonesia. Open Access Macedonian Journal of Medical Sciences, 10(A), 187–191.
Passali, D., Gregori, D., Lorenzoni, G., Cocca, S., Loglisci, M., Passali, F. M., & Bellussi, L. (2015). Foreign Body Injuries in Children: A Review. Acta Otorhinolaryngologica Italica, 35, 265–271.
PCNE. (2020). Classification for Drug related Problems (V9.1). Pharmaceutical Care Network Europe Association.
Pernica, J. M., Harman, S., Kam, A. J., Carciumaru, R., Vanniyasingam, T., Crawford, T., Dalgleish, D., Khan, S., Slinger, R. S., Fulford, M.,
Main, C., Smieja, M., Thabane, L., & Loeb, M. (2021). Short-Course Antimicrobial Therapy for Pediatric Community-Acquired Pneumonia: The SAFER Randomized Clinical Trial. JAMA Pediatr, 175(5), 475–482.
Pramudya, I. A. (2020). Identifikasi Drug Related Problems Penggunaan Antibiotik pada Penyakit Pneumonia Pasien Anak di Bangsal Rawat Inap Rumah Sakit Akademik Universitas Gadjah Mada. Universitas Gadjah Mada.
Rivetti, S., Romano, A., Mastrangelo, S., Attinà, G., Maurizi, P., & Ruggiero, A. (2023). Aminoglycosides-Related Ototoxicity: Mechanisms, Risk Factors, and Prevention in Pediatric Patients. Pharmaceuticals, 16(1353), 1–21. https://doi.org/10.3390/ph16101353
Same, R. G., Amoah, J., Hsu, A. J., Hersh, A. L., Sklansky, D. J., Cosgrove, S. E., & Tamma, P. D. (2021). The Association of Antibiotic Duration with Successful Treatment of Community-Acquired Pneumonia in Children. Journal of the Pediatric Infectious Diseases Society, 10, 267–273. https://doi.org/10.1093/jpids/piaa055
Sari, I. D. R., Hartini, L., & Mariati. (2016). Faktor-Faktor Yang Berhubungan Dengan Kejadian Pneumonia Pada Balita. Jurnal Meia Kesehatan, 9(2), 114–203.
Simanjuntak, E. S., Soleha, T. U., & Berawi, K. N. (2017). Kejadian Drug Related Problems (DRPs) pada Pasien Penderita Pneumonia Komuniti Berdasarkan Panduan PDPI (Perhimpunan Dokter Paru Indonesia) di Poliklinik Paru RSUD Jendral Ahmad Yani Periode April 2014−Maret 2015 Kota Metro. Medula, 7(5), 54–61.
Suci, L. N. (2020). Pendekatan Diagnosis dan Tatalaksana pneumonia pada anak. Jurnal Kedokteran Nanggroe Medika, 3(1), 30–38.
Suharjono, S., T, Y., Sumarno, S., & SJ, S. (2009). Studi Penggunaan Antibiotika Pada Penderita Rawat Inap Pneumonia (Penelitian Di Sub Departemen Anak Rumkital Dr. Ramelan Surabaya). Majalah Ilmu Kefarmasian, 6(3), 142–155. https://doi.org/10.7454/psr.v6i3.3443
Thom, K. A., Tamma, P. D., Harris, A. D., Dzintars, K., Morgan, D. J., Li, S., Pineles, L., Srinivasan, A., Avdic, E., & Cosgrove, S. E. (2019). Impact of a Prescriber-driven Antibiotic Time-out on Antibiotic Use in Hospitalized Patients. Clin Infect Dis, 68(9), 1581–1584.
Uranga, A., Artaraz, A., Bilbao, A., Quintana, J. M., Arriaga, I., Intxausti, M., Lobo, J. L., García, J. A., Camino, J., & España, P. P. (2020). Correction to: Impact of reducing the duration of antibiotic treatment on the long-term prognosis of community acquired pneumonia (BMC Pulmonary Medicine, (2020), 20, 1, (261), 10.1186/s12890-020-01293-6). BMC Pulmonary Medicine, 20(261), 1–8. https://doi.org/10.1186/s12890-020-01378-2
van Oers, J. A. H., Nijsten, M. W., & de Lange, D. W. (2018). Do we need new trials of procalcitonin-guided antibiotic therapy? A response. Critical Care, 22(83), 3–4. https://doi.org/10.1186/s13054-018-2008-y
WHO. (2022). Pneumonia in Children. World Health Organization.
Williams, D. J., Creech, C. B., Walter, E. B., Martin, J. M., Gerber, J. S., Newland, J. G., Howard, L., Hofto, M. E., Staat, M. A., Oler, R. E., Tuyishimire, B., Conrad, T. M., Lee, M. S., Ghazaryan, V., Pettigrew, M. M., Jr, V. G. F., Chambers, H. F., Zaoutis, T. E., Evans, S., … Study Team, T. D. 14-0079. (2022). Short- vs Standard-Course Outpatient Antibiotic Therapy for Community-Acquired Pneumonia in Children: The SCOUT-CAP Randomized Clinical Trial. JAMA Pediatr, 176(3), 253–261.